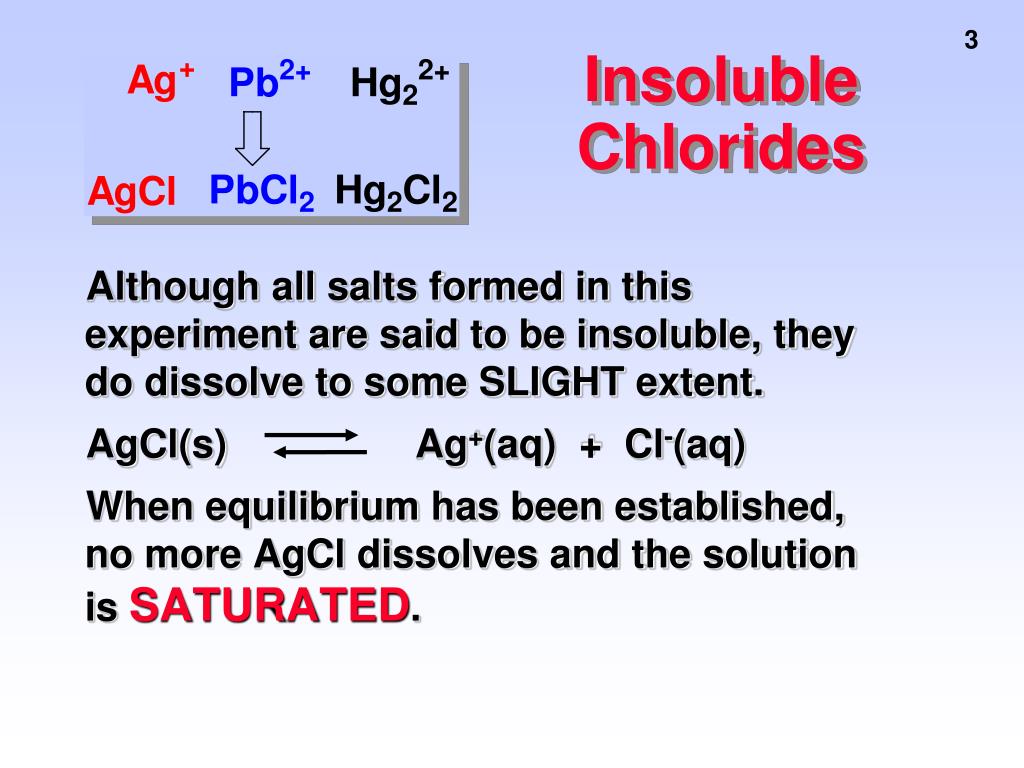
Chloride Water Insoluble . these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. a compound that is soluble in water forms an aqueous solution. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. 133 rows if i say one mole of sodium sulfate dissolves in water i am really meaning for every mole of sodium sulfate that dissolves,. silver chloride is one of the few chlorides that has a limited solubility. How to use solubility rules. Insoluble salts are made by precipitation. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. silver chloride (agcl) is an example of an insoluble salt. The water molecules cannot overcome the strong interionic forces that.
from www.slideserve.com
a compound that is soluble in water forms an aqueous solution. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). silver chloride is one of the few chlorides that has a limited solubility. 133 rows if i say one mole of sodium sulfate dissolves in water i am really meaning for every mole of sodium sulfate that dissolves,. silver chloride (agcl) is an example of an insoluble salt. Insoluble salts are made by precipitation. The water molecules cannot overcome the strong interionic forces that. soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble.
PPT PRECIPITATION REACTIONS Chapter 17 Part 2 PowerPoint Presentation
Chloride Water Insoluble Titration must be used if the reactants are soluble. Titration must be used if the reactants are soluble. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. 133 rows if i say one mole of sodium sulfate dissolves in water i am really meaning for every mole of sodium sulfate that dissolves,. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). Insoluble salts are made by precipitation. a compound that is soluble in water forms an aqueous solution. these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. silver chloride (agcl) is an example of an insoluble salt. The water molecules cannot overcome the strong interionic forces that. silver chloride is one of the few chlorides that has a limited solubility. soluble salts can be made by reacting acids with soluble or insoluble reactants. How to use solubility rules.
From www.toppr.com
Which of the following chloride is water insoluble Chloride Water Insoluble these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. Titration must be used if the reactants are soluble. a compound that is soluble in water forms an aqueous solution. silver chloride is one of the few chlorides that has a limited solubility. soluble. Chloride Water Insoluble.
From www.expii.com
Soluble vs. Insoluble — Comparison & Examples Expii Chloride Water Insoluble these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). silver chloride is one of. Chloride Water Insoluble.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Chloride Water Insoluble These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). Insoluble salts are made by precipitation. silver chloride (agcl) is an example of an insoluble salt. 133 rows if i say one mole of sodium sulfate dissolves in water i am really meaning for every mole of sodium sulfate that dissolves,. soluble. Chloride Water Insoluble.
From fphoto.photoshelter.com
science chemistry dissolution reaction silver chloride ammonium Chloride Water Insoluble These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). silver chloride is one of the few chlorides that has a limited solubility. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. 133 rows if i say one mole of sodium sulfate dissolves in water i. Chloride Water Insoluble.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Chloride Water Insoluble a compound that is soluble in water forms an aqueous solution. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. Insoluble salts are made by precipitation. soluble salts. Chloride Water Insoluble.
From mungfali.com
Sodium Chloride Dissolved In Water Chloride Water Insoluble silver chloride is one of the few chlorides that has a limited solubility. silver chloride (agcl) is an example of an insoluble salt. a compound that is soluble in water forms an aqueous solution. Titration must be used if the reactants are soluble. soluble salts can be made by reacting acids with soluble or insoluble reactants.. Chloride Water Insoluble.
From www.chegg.com
Solved Which chloride salt is insoluble in cold water but Chloride Water Insoluble 133 rows if i say one mole of sodium sulfate dissolves in water i am really meaning for every mole of sodium sulfate that dissolves,. soluble salts can be made by reacting acids with soluble or insoluble reactants. How to use solubility rules. Insoluble salts are made by precipitation. Titration must be used if the reactants are soluble.. Chloride Water Insoluble.
From www.nagwa.com
Question Video Using the Water Solubility Rules to Determine Which Chloride Water Insoluble These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). a compound that is soluble in water forms an aqueous solution. these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. soluble salts can be made by reacting acids with. Chloride Water Insoluble.
From depositphotos.com
Test Chloride Infographic Diagram Showing Laboratory Experiment Chloride Water Insoluble silver chloride (agcl) is an example of an insoluble salt. The water molecules cannot overcome the strong interionic forces that. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). soluble salts can be made by reacting acids with soluble or insoluble reactants. How to use solubility rules. A precipitate is also formed. Chloride Water Insoluble.
From www.pinterest.com.mx
Solubility Rules Chart for Chemistry Classroom Teaching chemistry Chloride Water Insoluble silver chloride is one of the few chlorides that has a limited solubility. Insoluble salts are made by precipitation. a compound that is soluble in water forms an aqueous solution. soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. The water molecules cannot overcome. Chloride Water Insoluble.
From www.slideserve.com
PPT PRECIPITATION REACTIONS Chapter 17 Part 2 PowerPoint Presentation Chloride Water Insoluble silver chloride (agcl) is an example of an insoluble salt. these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. The water molecules cannot overcome the strong interionic forces that. soluble salts can be made by reacting acids with soluble or insoluble reactants. These include. Chloride Water Insoluble.
From www.chegg.com
Solved The formation of waterinsoluble silver chloride is Chloride Water Insoluble The water molecules cannot overcome the strong interionic forces that. How to use solubility rules. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. silver chloride is one of the few chlorides that has a limited solubility. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4).. Chloride Water Insoluble.
From fphoto.photoshelter.com
science chemistry dissolution reaction silver chloride ammonium Chloride Water Insoluble a compound that is soluble in water forms an aqueous solution. Titration must be used if the reactants are soluble. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). How to use solubility rules. soluble salts. Chloride Water Insoluble.
From studymind.co.uk
ᐉ Solubility Rules Insoluble & Soluble Salts Making Chloride Water Insoluble These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). 133 rows if i say one mole of sodium sulfate dissolves in water i am really meaning for every mole of sodium sulfate that dissolves,. soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if. Chloride Water Insoluble.
From www.oakabooks.co.uk
KS4/GCSE Making an Insoluble Salt Revision Resource For Dyslexics Chloride Water Insoluble A precipitate is also formed when sodium carbonate is added to a sample of hard water,. silver chloride is one of the few chlorides that has a limited solubility. The water molecules cannot overcome the strong interionic forces that. a compound that is soluble in water forms an aqueous solution. silver chloride (agcl) is an example of. Chloride Water Insoluble.
From www.youtube.com
Step 2 GroupI cations. Adding hot water the insoluble chlorides YouTube Chloride Water Insoluble The water molecules cannot overcome the strong interionic forces that. How to use solubility rules. silver chloride is one of the few chlorides that has a limited solubility. 133 rows if i say one mole of sodium sulfate dissolves in water i am really meaning for every mole of sodium sulfate that dissolves,. Insoluble salts are made by. Chloride Water Insoluble.
From www.doubtnut.com
Name (a) a chloride which is insoluble in cold water but dissolves Chloride Water Insoluble Titration must be used if the reactants are soluble. Insoluble salts are made by precipitation. soluble salts can be made by reacting acids with soluble or insoluble reactants. silver chloride is one of the few chlorides that has a limited solubility. These include lead chromate (pbcr 2 o 4) and barium chromate (bacr 2 o 4). 133. Chloride Water Insoluble.
From www.tradeindia.com
80 Pure 7.5 Ph 74.5 Water Insoluble Powder Form Calcium Chloride Chloride Water Insoluble silver chloride (agcl) is an example of an insoluble salt. Insoluble salts are made by precipitation. A precipitate is also formed when sodium carbonate is added to a sample of hard water,. these attractions play an important role in the dissolution of ionic compounds in water, which will be later discussed in chapter 14. These include lead chromate. Chloride Water Insoluble.